For many people, radium brings to mind Marie Curie and her historic discoveries and the dangers they posed. But radium is found all around—in rocks, sediments, and water— in such small amounts it cannot harm us. Several isotopes of radium exist naturally in the environment. They decay at different rates, and scientists like Cátia Ehlert von Ahn from the Leibniz Centre for Tropical Marine Research (ZMT) can use them to investigate different processes both short term (like porewater exchange) and long term (like ocean water mass transport). Let’s take a look at how she is putting radium to work onboard the Sonne!
Many scientists quantify elements and chemical species in terms of concentration—how much of x per unit y. However, radioactive elements are constantly decaying, and are thus often quantified in terms of activity, or the rate of decay. In the case of radium, Cátia measures four different isotopes of radium with half-lives ranging from 3.4 days to 1600 years.
Since radium is found at such low levels in the environment, she needs to process a lot of water to measure radium activity. At the surface, she uses a submersible pump to filter about 600 liters of water. For deeper depths, she takes advantage of the in-situ pumps; these are powerful tools for chemical oceanography, as they can be lowered to specific depths at which they pump over 1000 liters of water. Both pump systems are equipped with filters/fibers coated with manganese oxide to allow adsorption of the radium from the water.

Still on the ship, Cátia attaches the filters to a machine called a radium decay coincidence counter (RaDeCC). The RaDeCC pumps helium through the filters to transport radon (Rn), generated from the captured radium, through a scintillation cell where the Rn decay is counted. This provides the activity measurements Cátia uses to evaluate how much radium is in the water.
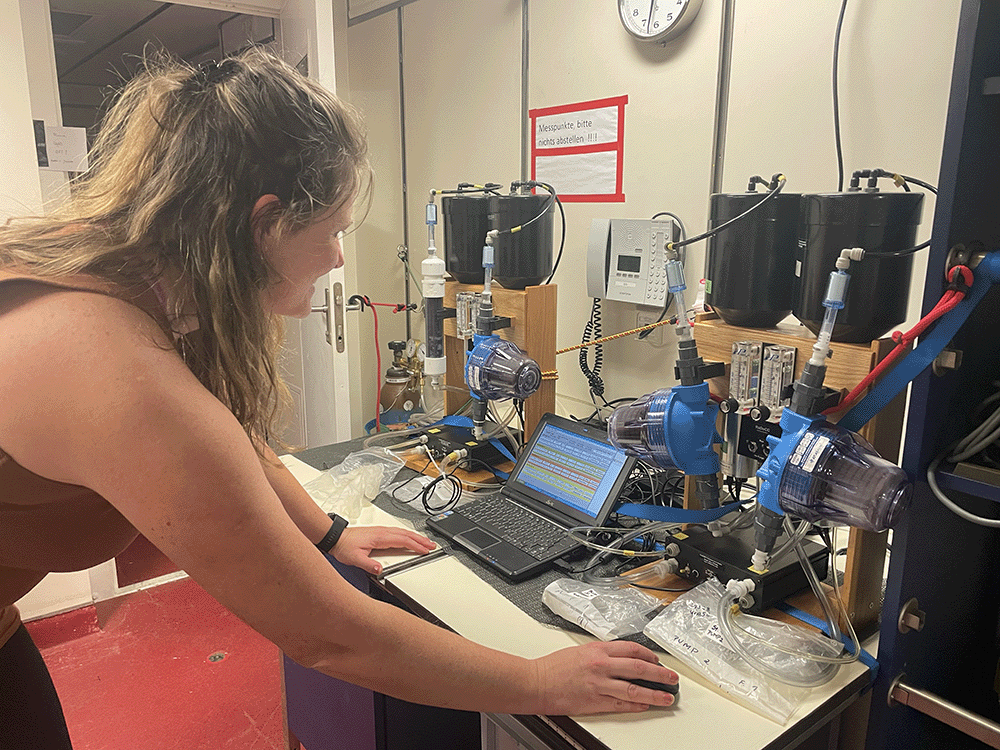
The measurements Cátia makes onboard only capture the decay of short-lived radium isotopes. She will take other measurements back at ZMT of each sample to measure the activities of radium isotopes with longer half-lives.
Piecing together the activities of different radium isotopes at various locations and depths along the cruise transect will enable the identification and quantifications of element sources from continental margins to the open ocean as well as provide insights into water mass movement, which has major implications for the transport of chemical species. A little radium goes a long way when it comes to understanding element sources!
By Charlotte Eckmann based on interviews with Cátia Ehlert von Ahn. Cátia Ehlert von Ahn provided edits.